PANACEA
PAN Alimentary Cancer Exhaled breath Analysis
Our funders

NIHR Clinical Research Network portfolio adoption: CPMS ID: 60056
Sponsor: Imperial College London.
Funding: National Institute for Health and Care Research (NIHR i4i & OLS Cancer Mission)

Background
In the UK, there are over 75,000 new cases annually of gastrointestinal cancers (oesophageal, gastric, pancreatic, liver and colorectal). The overall 5-year survival for these cancers is poor and even lower in deprived areas. Symptoms related to gastrointestinal cancers are non-specific, therefore the decision to refer for investigations is challenging. Currently, the cancer yield of urgent and non-urgent referral pathways is 4.4-5.0% and 0.1-1.7% respectively.
Breath analysis offers a simple, quick and non-invasive method to detect volatile organic compounds (VOCs) in breath that are specific to gastrointestinal cancers. The triage test would maximise patient compliance, particularly in certain ethnic groups and deprived areas.
PANACEA
Aims:
1. Can we validate VOCs in the detection of multi-gastrointestinal cancers?
2. How well does a single breath test perform in the detection of multi-gastrointestinal cancers?
3. What is the optimal strategy to implement the breath test in primary care?
4. Would a triage breath test reduce costs in NHS diagnostic pathways?
Methods:
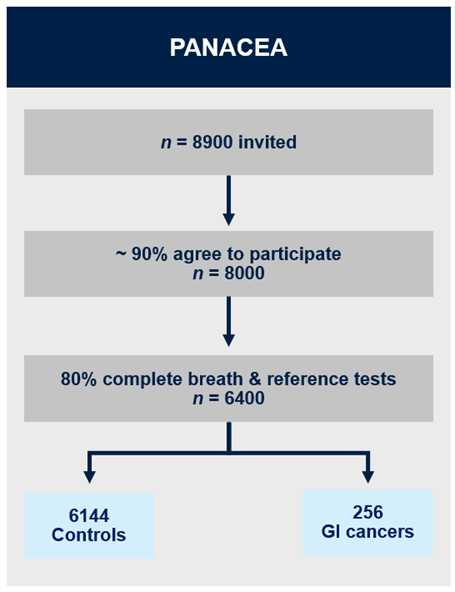
PANACEA is a prospective multicentre clinical study. At least 8,000 individuals with suspected cancer, referred from primary care along upper and lower gastrointestinal cancer pathways, will be recruited from 40 centres. We aim to have 256 cancers and 6,144 controls with complete dataset (reliable breath data and results of reference test). Breath samples will be collected on thermal desorption tubes which will be analysed using gas chromatography-mass spectrometry. We will examine diagnostic accuracy of the breath test to detect cancer and estimate the most likely cancer from upper and lower gastrointestinal referral pathways.
The views of key stakeholders will be captured through interviews, workshops and digital platforms and their feedback will be integrated into strategic decision-making, public engagement and awareness campaigns.
Multiple implementation models will be co-designed and tested for breath test delivery in primary care. Process evaluation will be conducted across three sites to determine the strengths and weaknesses of the models. Guidance on best practice in breath test delivery for primary care will be developed.
Lastly, a cost-effectiveness and budget impact analysis will be performed to understand the affordability and financial impact on the NHS.
Contact
For more information relating to the study, or if you are a healthcare professional interested in your centre participating in this study, please get in touch with Michael Fadel, NIHR Doctoral Fellow, via email: m.fadel@imperial.ac.uk
