AROMA
Augmented Response of VOlatile BioMarkers in the Assessment of Oesophagogastric Adenocarcinoma
Our funders

ClinicalTrials.gov Identifier:
ClinicalTrials.gov website:
https://clinicaltrials.gov/study/NCT05412758
NIHR Clinical Research Network portfolio adoption:
CPMS ID: 50774
Sponsor: Imperial College London
Funding: Cancer Research UK programme

Background
Cancers of the oesophagus and stomach are common and have low survival rates. Patients with early oesophagogastric cancer often have non-specific symptoms that can be associated either with cancer or, more often, a common non-cancerous condition. As the cancer grows, patients may suffer from difficulty swallowing, weight loss and pain.
These are known as ‘red flag’ symptoms and are usually found in patients with advanced incurable cancers. Currently only patients with ‘red flag’ symptoms are urgently referred for diagnostic testing, usually by endoscopy. Patients with non-specific symptoms are frequently not referred for further tests because the tests could be harmful or unnecessary. Testing patients with non-specific symptoms utilises substantial NHS resources and can lead to patient anxiety.
We have reported a distinct VOC profile associated with the exhaled breath, gastric content and urine from patients with oesophagogastric cancer. We have developed a cancer detection model with internal validation in 225 patients with an area under the receiver operator characteristic curve (AU-ROC) of 0.92 [Ann Surg. 2015, 262 (6), 981-90]. The biomarker panel was externally validated in a multicentre study of 325 participants with an AU-ROC of 0.85 [JAMA Oncol. 2018, 4 (7), 970-976]. A further study with 300 patients expanded the biomarker panel using a different analytical platform and a thermal desorption (TD) breath collection method which allowed for improved VOC storage and robust transport. This study had an AU-ROC of 0.90.
In this programme, we aim to increase VOC production to improve cancer detection in exhaled breath. This programme will be delivered in a laboratory setting, as well as in a series of clinical trials. By using simple nutrients, including sugars and fats, we aim to stimulate the cancer cells and/or associated bacteria to produce more VOCs. The nutrients will be administered via an oral drink, following which serial breath samples will be taken. The aim is to improve the efficacy of our breath test in later clinical trials.
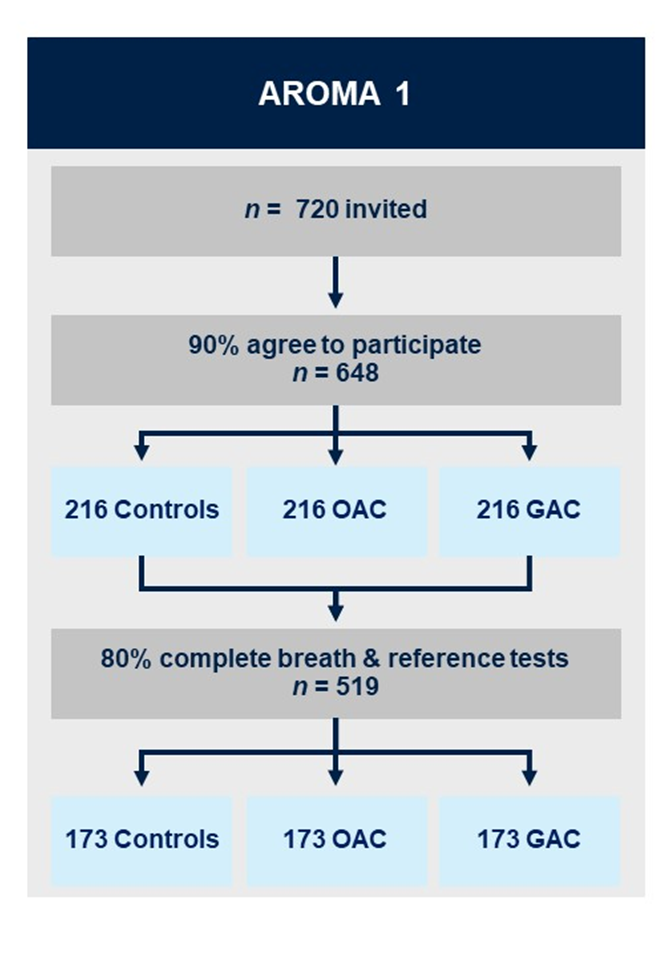
AROMA1 (currently recruiting)
Aim:
Construct a cancer detection model based on the augmented breath test, and to refine the baseline breath test.
Methods:
AROMA1 is a prospective multicentre prediction study with internal validation. A total of 648 patients will be recruited for the development of an augmented cancer detection model. Three groups of patients will be recruited, each comprised of 216 patients with: (i) oesophageal/ gastro-oesophageal junction adenocarcinoma; (ii) gastric adenocarcinoma; and (iii) benign conditions / normal gastrointestinal tract with upper gastrointestinal symptoms.
Each patient will provide a baseline exhaled breath sample. Thereafter, each patient will consume the oral stimulant drink and then provide a further breath sample at 15 minutes after consumption of the drink. Breath will be collected using a standardised protocol and transported using TD tubes to the Imperial College London VOC Laboratory. The breath samples will be analysed using mid-polar, polar and two-dimensional gas chromatography-time of flight mass-chromatography (GC-MS-TOF) for VOC detection.
Participating Centres:

AROMA Bioresource (currently recruiting)
Aim:
To characterise a comprehensive volatolomic, transcriptomic, metabolomic and metagenomic profile of matched samples of exhaled breath, tissue (adenocarcinoma and controls) and biofluids (gastric fluid, saliva, urine and blood).
Methods:
AROMA Bioresource is a cross-sectional observational study. A total of 335 patients will be recruited to provide samples for the proposed biobank. Four groups will be recruited: (i) oesophageal/gastro-oesophageal junction adenocarcinoma (110 patients); (ii) gastric adenocarcinoma (75 patients); (iii) oesophageal control patients- benign conditions/ normal gastrointestinal tract with upper gastrointestinal symptoms (75 patients); (iv) gastric control patients – benign conditions/normal gastrointestinal tract with upper gastrointestinal symptoms (75 patients).
Participating centres:

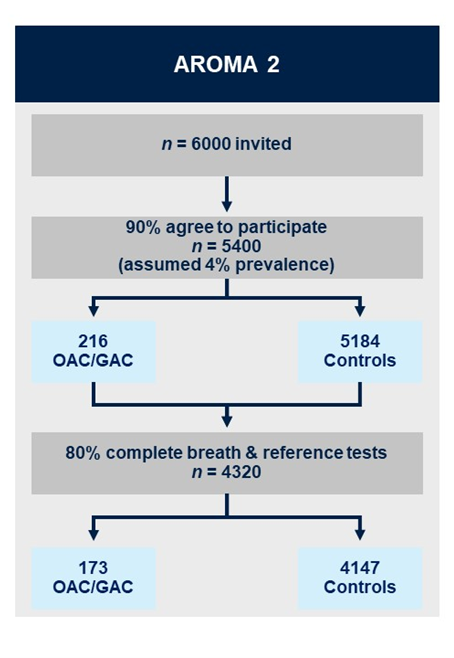
AROMA2 (recruitment planned to start 2025)
Aims:
1. Validate the breath test to detect oesophagogastric adenocarcinoma in patients with upper gastrointestinal symptoms.
2. Perform a cost-benefit analysis to assess the value of using the breath test in primary care.
3. Consider the barriers to adoption and develop mitigation strategies for scaling-up in clinical practice.
Methods:
AROMA2 is multicentre, triple-blind external validation study. As the baseline and augmented breath tests happen prior to endoscopy and histopathology results being available, the definitive diagnosis of cancer/no-cancer will not be available at the time of breath collection/analysis to: (i) the patient; (ii) the research nurse obtaining the exhaled breath (index test) or (iii) the VOC analyst in the laboratory or the statistician/bioinformatician classifying the VOC profile into cancer/no-cancer. Additionally, the endoscopists and histopathologists performing the reference test will not have access to the results of the breath test at any point. The augmented breath test will be conducted at the points identified from the work in AROMA1. Breath samples will be collected following a standardised procedure and will be analysed using TD-GC-TOF-MS.
Participating Centres:

Contact
For more information relating to the study, or if you are a healthcare professional interested in your centre participating in this study, please get in touch with Ayushi Pabari, our AROMA Trial Manager, via email: aroma@imperial.ac.uk.
