AVOCADO
Augmentation of Volatile Biomarkers of Oesophageal and Gastric AdenoCArcinoma from the Tumour LipiDOme
Our funders


ClinicalTrials.gov Identifier:
NCT05600608
ClinicalTrials.gov website:
https://classic.clinicaltrials.gov/ct2/show/NCT05600608
Sponsor: Imperial College London.
Funding: Medical Research Council (UK), BASO, RCS England


Background
In the UK, nearly 10,000 people die each year from cancers of the oesophagus and stomach and this number is predicted to rise. Early cancer detection is essential to improving survival. The breath of oesophageal adenocarcinoma (OAC) patients contains chemical compounds that indicate cancer. The Imperial College London VOC Laboratory has developed an oesophagogastric cancer detection model based on volatile organic compounds (VOCs) present in baseline exhaled breath samples, with an area under the receiver operator characteristic curve (AU-ROC) of 0.92 [Ann Surg. 2015, 262 (6), 981-90].
This study aims to understand from ‘where, why and how’ these compounds are produced by specifically interrogating the reprogrammed tumour lipidome, since many of the terminal VOCs identified in breath are downstream products of lipid breakdown. The kinetics of VOC production from lipids will be investigated through a series of in vitro and ex vivo experiments, which will ultimately translate to an augmented breath test. This will benefit patients in the short term through early cancer detection, which benefits patients in the long term through improved overall survival.
AVOCADO (recruitment complete)
Participating Centres:

Aim:
To evaluate the origins of oesophagogastric cancer associated VOCs by interrogating the reprogrammed tumour lipidome, and to augment the production of these diagnostic VOCs by targeting their pathways of production.
Methods:
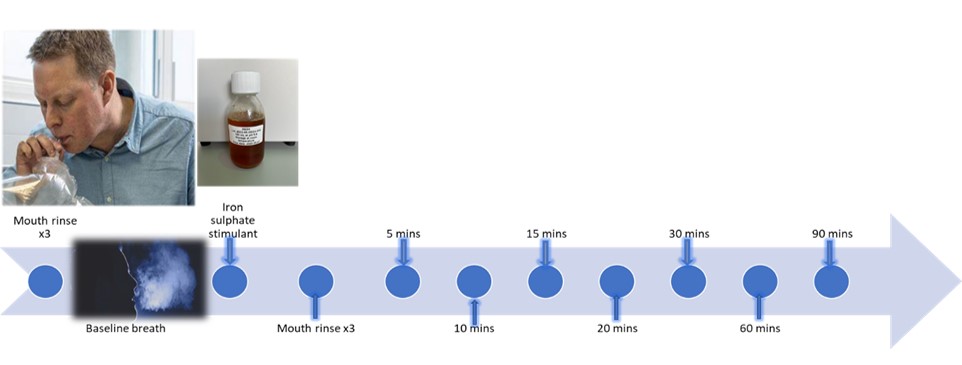
The AVOCADO study is a prospective, single centre, interventional study. A total of 100 patients will be recruited for development of the augmented cancer detection model. Two groups of patients will be recruited at a 1:1 ratio comprising of: (i) confirmed oesophageal / gastro-oesophageal junction adenocarcinoma and (iii) benign conditions / normal gastrointestinal tract with upper gastrointestinal symptoms. Breath samples will be collected using a standardised procedure and will be transported using thermal desorption (TD) tubes to the Imperial College London VOC Laboratory. Breath samples will be analysed using mid-polar, polar and two-dimensional gas chromatography-time of flight mass-chromatography (GC-MS-TOF) for VOC detection.
Participant involvement flow chart:
Contact
For more information relating to the study, please get in touch with Anuja Mitra, the lead for this study, via email: anuja.mitra06@imperial.ac.uk.
