HOPE
HOspital versus Primary care breath test Environment study
Our funders

ClinicalTrials.gov Identifier: NCT06611293
ClinicalTrials.gov website:
https://clinicaltrials.gov/study/NCT06611293
Sponsor: Imperial College London
Background
The use of a breath test as a triage tool for cancer detection is a simple, non-invasive method that could help identify high-risk patients who should be referred for investigations at an earlier stage. This would increase the proportion of appropriate referrals from Primary care and improve adherence to National Institute for Clinical Health and Excellence (NICE) guidance. If a GP is presented with a patient with symptoms that do not prompt referral under NICE guidelines, they would no longer need to watch-and-wait to see if their symptoms worsen, but could instead offer a breath test immediately. Breath samples would be collected and sent to a regional laboratory for analysis. A positive result would warrant immediate referral for further specialised investigations, whilst a negative test would permit the GP to reassure the patient and offer re-testing if symptoms persist.
The MAGIC (Methodological Approaches towards a GastroIntestinal Cancer test) study was performed to assess whether breath testing is feasible in primary care. In this study, 1,002 patients took part in the breath test in primary care. Ninety-nine percent of patients found the breath test very easy or easy to perform, and it was demonstrated that breath testing is feasible in primary care.
However, the potential for an individual’s exhaled breath volatile organic compound (VOC) profile to change when measured across Primary and Secondary care sites has not yet been investigated. Through the HOPE study, the intention would be to determine whether exhaled breath VOC profiles in Primary care differ from Secondary care. This would support the clinical translation of breath research outcomes from studies performed in Secondary care, despite the breath test being intended for use in Primary care.
HOPE (currently recruiting)
Aims:
Assess any differences in an individuals’ exhaled breath VOC profile when measured across Primary and Secondary care sites.
Methods:
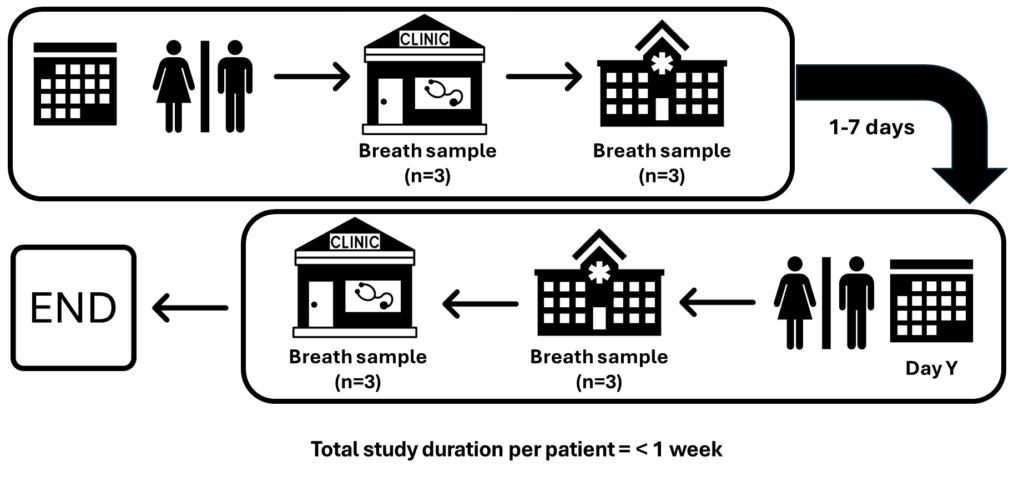
HOPE is a single-centre observational study at Imperial College Healthcare Trust with 80 participants. Participants will perform breath testing in Primary care followed by Secondary care on Day 1. At each location, participants will be asked to perform three breath tests (15 minutes apart). The environmental air will also be sampled for quality control. This entire process will be repeated in the reverse order (i.e. breath testing in Secondary care followed by Primary care on Day 2). Participants will be asked to maintain a clear fluid diet for a minimum of six hours prior to breath collection on both Day 1 and Day 2 of sampling.
An exploratory analysis will be performed to identify and exclude contaminated samples. Linear mixed models will be fitted for each compound’s measurements to estimate components of variation (between individuals, occasions and repeat samples), and confirm that there is no effect of ordering (i.e. hospital to GP vs GP to hospital). The observed differences will be compared with the clinically acceptable limits for each VOC to identify the proportion of GP to hospital differences that fall within these limits.
Study design:
Participating centres:

Contact
For more information relating to the study, please get in touch with Mr Mohamed Abulazayem, via email: hope-study@imperial.ac.uk
