NEED
Non-Invasive Testing for Early OEsophageal Cancer and Dysplasia
Our funders

ClinicalTrials.gov Identifier:
NCT04001478
ClinicalTrials.gov website:
https://classic.clinicaltrials.gov/ct2/show/NCT04001478
NIHR Clinical Research Network portfolio adoption:
CPMS ID: 42082
Sponsor: Imperial College London.
Funding: Guts UK

Background
Oesophageal cancer is the eighth commonest cancer worldwide, the sixth leading cause of cancer deaths both worldwide and in the UK, and accounts for approximately 8000 deaths per year in the UK. In the Western world, adenocarcinoma of the oesophagus is the predominant histological subtype of oesophageal cancer. Prognosis of oesophageal cancer is poor, with a 5-year survival in the UK of under 20%. The current diagnostic pathway for suspected oesophageal cancer is based on referral for endoscopy via the two-week-wait pathway, which is based on age and “red-flag symptoms” which typically indicate advanced incurable disease. It is well recognised that the two-week-wait criteria are neither sensitive nor specific, with only 4% of patients referred on this pathway being subsequently diagnosed with an upper gastrointestinal (GI) cancer at endoscopy.
A further challenge for general practitioners is that early oesophageal cancer typically presents either with no symptoms, or non-specific upper GI symptoms such as reflux and dyspepsia, more commonly seen in benign disease. Investigating all patients with non-specific upper GI symptoms with endoscopy is neither feasible nor appropriate given the invasive nature of endoscopy. Primary care physicians need a test to triage patients with non-specific upper GI symptoms into those who can be managed expectantly or investigated non-urgently and those who need urgent investigation with endoscopy. It is our intention to develop the breath test to be used for this purpose in primary care.
Pilot data from the NEED-1 study (Non-Invasive Testing for Early Oesophageal Cancer and Dysplasia) has generated a breath-VOC biomarker model that was able to differentiate between cancer and non-cancer, which will be validated in NEED-2.
NEED2 (currently recruiting)
Aim:
To refine and validate a breath VOC model in a large cohort of patients with early adenocarcinoma of the oesophagus, in a multi-centre clinical study.
Methods:
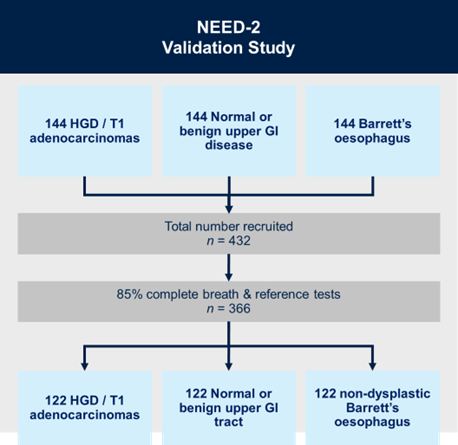
NEED-2 is a prospective, multi-centre, diagnostic study with internal validation. We will recruit: (i) 144 patients with known T1 stage oesophageal adenocarcinoma or high-grade dysplasia (the precursor to oesophageal adenocarcinoma); (ii) 144 patients with a normal upper GI tract or benign upper GI disease; and (iii) 144 patients with Barrett’s oesophagus. Breath samples will be collected using a standardised protocol, and will be analysed using mid-polar and polar gas chromatography-time of flight mass-chromatography (GC-MS-TOF) for VOC detection.
Participating Centres:

Contact
For more information relating to the study, or if you are a healthcare professional interested in your centre participating in this study, please get in touch with Katja Christodoulou, via email: k.christodoulou@imperial.ac.uk.
