VAPOR
Volatile Organic Compound Assessment in Pancreatic Ductal AdenOcaRcinoma
Our funders

ClinicalTrials.gov Identifier:
NCT05727020
ClinicalTrials.gov website:
https://clinicaltrials.gov/ct2/show/NCT05727020
NIHR Clinical Research Network portfolio adoption:
CPMS ID: 53691
Sponsor: Imperial College London
Funding: Pancreatic Cancer UK

Background
‘Red flag’ symptoms, including obstructive jaundice, frequently indicate advanced and incurable disease. By contrast, the most common initial symptoms of pancreatic ductal adenocarcinoma such as indigestion, loss of appetite, fatigue, and ‘feeling different’ are found to be just as common in patients without the disease.
Existing pathways for the detection of pancreatic ductal adenocarcinoma use cross-sectional imaging (CT and/or MRI scans) with endoscopy for biopsy-confirmation. This diagnostic approach is not a viable option for non-specific and often intermittent symptoms as they would lead to over-investigation and consume significant NHS resources, with the added burden of exposing patients to ionising radiation and invasive procedures.
There is a need for an intermediate test to streamline referrals in patients with suspected pancreatic ductal adenocarcinoma to receive specialised investigations. Our proposed solution is a breath test to act as a triage tool in primary care.
We carried out a discovery study to identify potential biomarkers to detect pancreatic ductal adenocarcinoma [Br. J. Surg. 2018, 105, 1493-1500]. This showed a distinctive profile of VOCs for pancreatic ductal adenocarcinoma in the exhaled breath. The area under the receiver operator characteristic (AU-ROC) curve was 0⋅901 to distinguish between pancreatic cancer and benign pancreatic conditions.
VAPOR1 (currently recruiting)
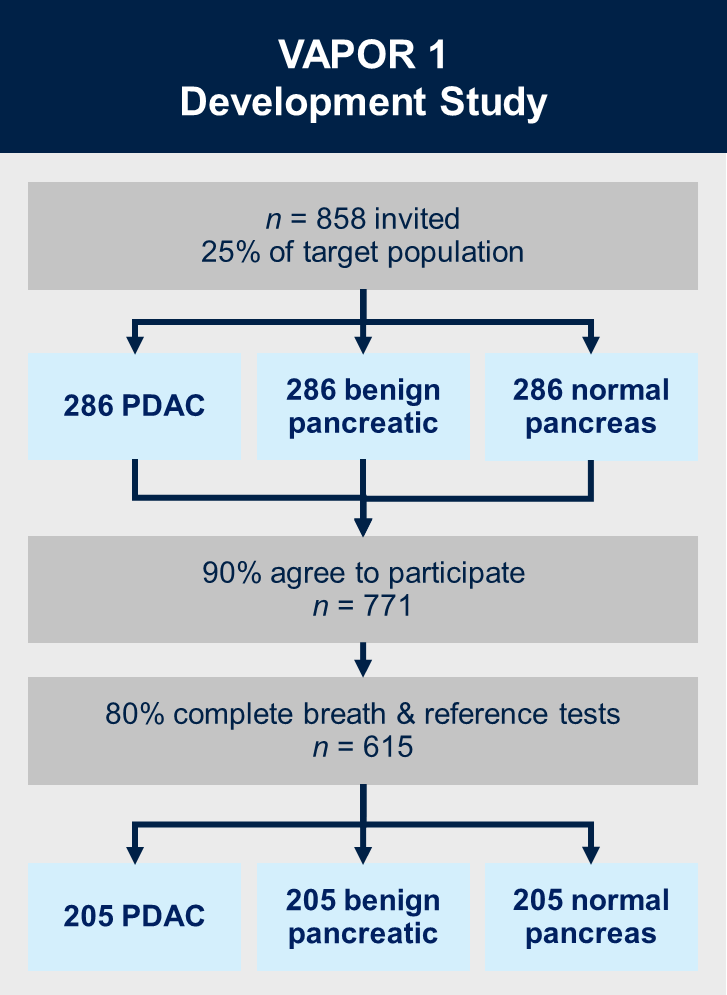
Aims:
1. Validate biomarkers in our discovery study
2. Develop a diagnostic model for detection of pancreatic ductal adenocarcinoma based on identified biomarkers
Methods:
VAPOR1 is a prospective, multi-centre diagnostic study with internal validation. We will recruit 771 patients with known pancreatic cancer, benign pancreatic conditions (new-onset diabetes and chronic pancreatitis) and healthy controls with a normal pancreas on imaging. Breath will be collected using a standardised procedure and transported using thermal desorption (TD) tubes to the Imperial College London VOC Laboratory. VOCs will be analysed using mid-polar, polar and two-dimensional gas chromatography-time of flight mass-chromatography (GC-MS-TOF).
Participating Centres:

VAPOR Bioresource (currently recruiting)
Aim:
To establish a characterised platform of volatolomic, transcriptomic, metabolomic and metagenomic datasets from matched patient samples including exhaled breath, pancreatic ductal adenocarcinoma tissue, adjacent histologically-normal pancreatic tissue, and biofluids (saliva, urine, blood and duodenal fluid).
Methods:
We will recruit 96 patients with chemotherapy-naive pancreatic ductal adenocarcinoma and 96 control non-cancer patients. The control group includes patients with benign conditions such as intraductal papillary mucinous neoplasms, pancreatic mucinous cystic neoplasms, chronic pancreatitis and large duodenal adenomas necessitating pancreatic resection. A series of profiling and experimental mechanistic studies will determine the origin of VOC biomarkers in patients with pancreatic ductal adenocarcinoma.
Participating centres:

VAPOR2 (recruitment planned to start 2025)
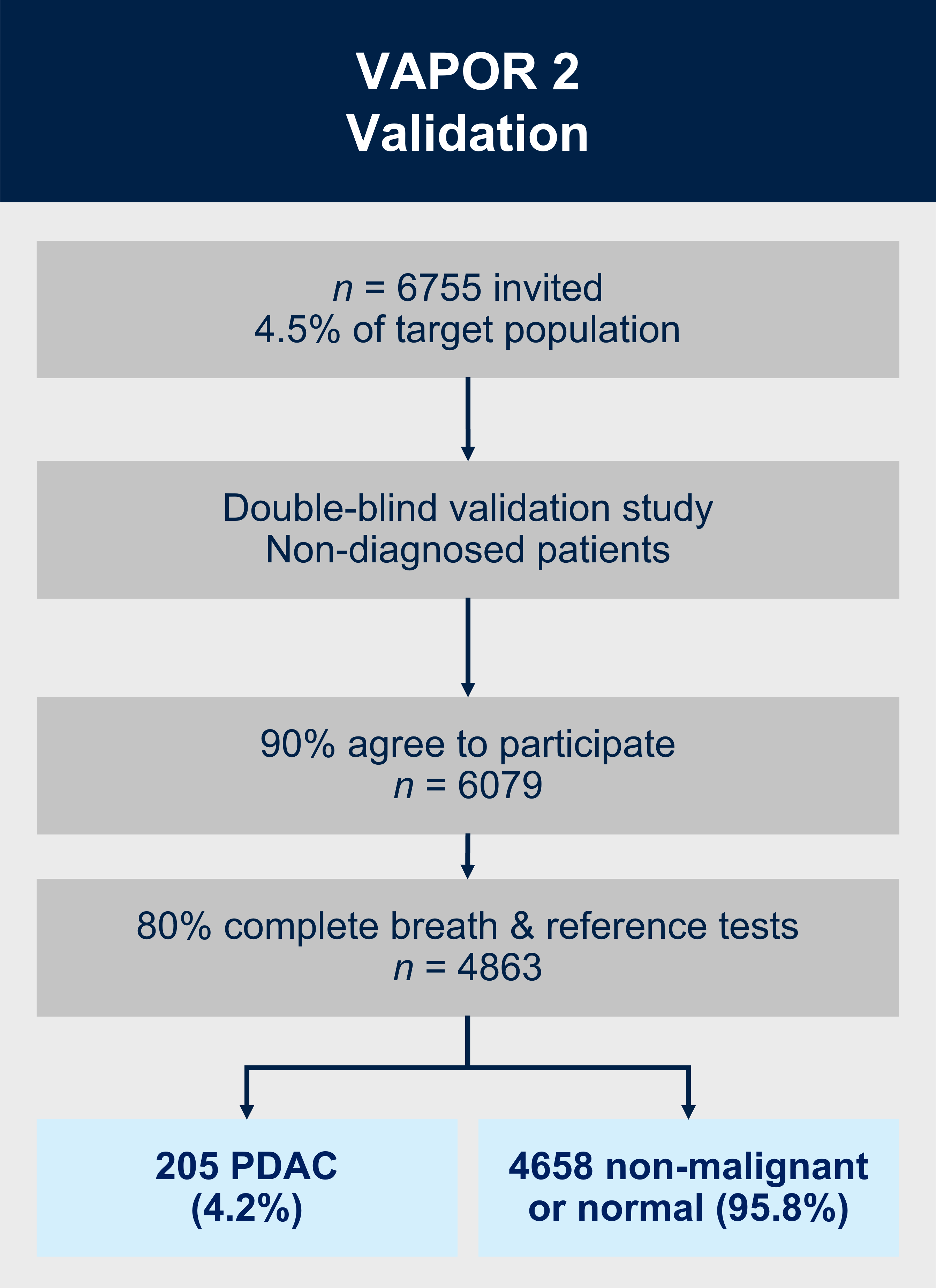
Aims:
1. Validate the breath test to detect pancreatic ductal adenocarcinoma in patients with upper gastrointestinal symptoms.
2. Perform a cost-benefit analysis to assess the value of using the breath test in primary care.
Methods:
VAPOR2 is a multicentre double-blind external validation study. We will recruit 6079 patients with gastrointestinal symptoms referred along the urgent suspected cancer referral pathway. As the breath test will happen prior to the patient or clinician obtaining the results of the imaging/endoscopy and histopathology, the definitive diagnosis of cancer/no-cancer will not be available at the time of breath collection or analysis to: (i) the patient; (ii) the research nurse obtaining the exhaled breath (index test); or (iii) the VOC analyst in the laboratory or the statistician/bioinformatician classifying the VOC profile into cancer/no-cancer. Additionally, the radiologist reporting the reference test will not have access to the results of the breath test at any point. The breath samples will be collected in a similar method to VAPOR1 and will be analysed using TD-GC-TOF-MS.
Contact
For more information relating to the study, or if you are a healthcare professional interested in your centre participating in this study, please get in touch with Emma Austin, our VAPOR Trial Manager, via email: vapor@imperial.ac.uk
