ViSON
Volatile Organic Compounds as Breath Biomarkers in Squamous Oesophageal Neoplasms
Our funders

ClinicalTrials.gov Identifier:
NCT06169163
ClinicalTrials.gov website:
https://clinicaltrials.gov/study/NCT06169163
NIHR Clinical Research Network portfolio adoption:
CPMS ID: 59207
Sponsor: Imperial College London.
Funding: National Institute of Health and Care Research (NIHR)

Background
Oesophageal Squamous Cell Carcinoma (OSCC) affects around 2000 patients in the UK every year. It is often detected at an advanced stage, resulting in poor overall survival (5-year survival less than 20%). Early detection can improve 5-year survival to over 70%, however early detection of OSCC is challenging for many reasons. Firstly, early disease symptoms are non-specific, which patients often overlook. Secondly, ‘Alarm’ symptoms such as weight loss or difficulty swallowing are signs of advanced stage, and thirdly there are no national screening guidelines. Furthermore, endoscopy is currently the only available test to detect OSCC, however it is invasive, expensive, and uncomfortable for patients.
Developing a breath test would provide an ideal triage test for patients with non-specific symptoms as it is non-invasive, simple to perform, cost-effective and highly acceptable to patients. The breath test is based on identifying volatile organic compounds (VOCs) released in breath. General Practitioners could offer the test to patients with non-specific symptoms, and those who test positive would be referred for an urgent endoscopy, whilst those who test negative could be reassured or offered re-testing at a later stage.
ViSON (currently recruiting)
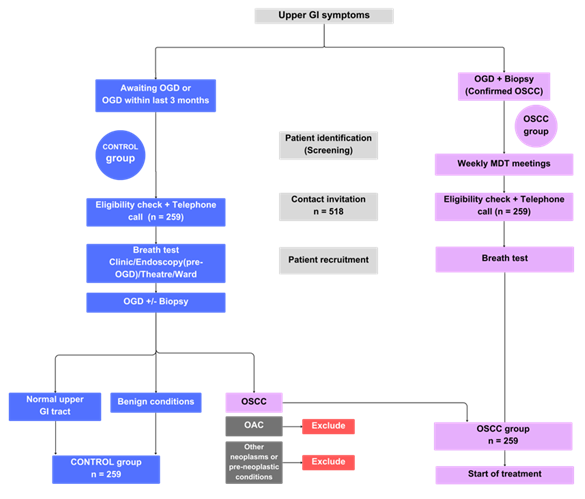
Aims:
1. To profile the VOCs in exhaled breath of patients with OSCC and non-cancer controls.
2. Develop a breath test for the detection of OSCC.
3. Evaluate the effect of known confounding variables on the target VOCs in OSCC.
Methods:
ViSON is a multi-centre profiling case-control study with prospective data collection. The recruitment target is a 518 participants, divided equally between the cancer and non-cancer group (OSCC group = 259; non-cancer group = 259).
Breath samples are collected using standardised procedures and are transported using thermal desorption (TD) tubes to the Imperial College London VOC Laboratory. Breath samples will be analysed using mid-polar, polar, and two-dimensional gas chromatography-time of flight mass-chromatography (GC-MS-TOF) for VOC detection.
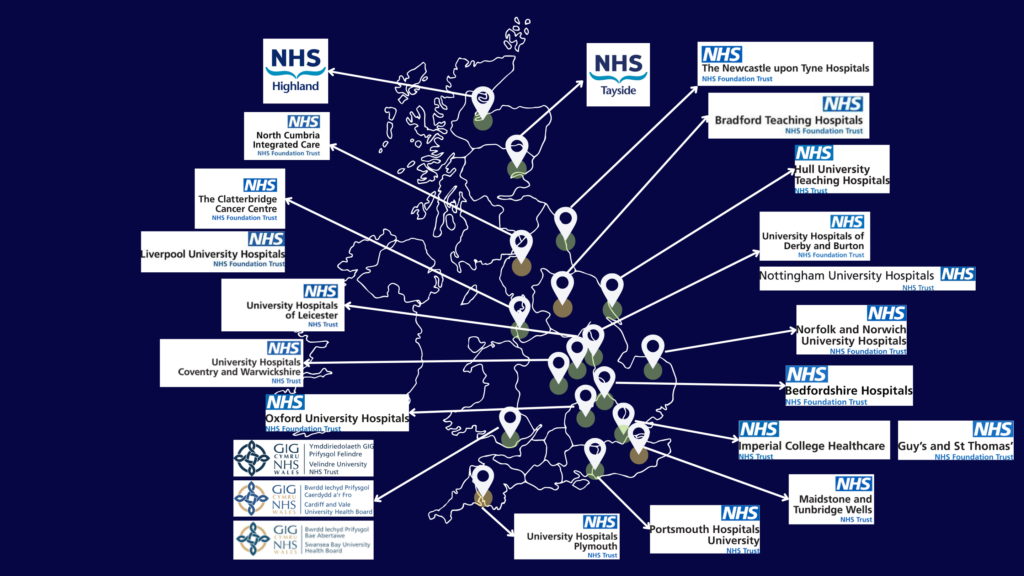
Participating Centres:
Contact
For more information relating to the study, please get in touch with Ms Sameera Sharma, via email: vison@imperial.ac.uk.

