VOCAL
Volatile Organic Compound Assessment of Liver disease
Our funders

ClinicalTrials.gov Identifier:
NCT04620538
ClinicalTrials.gov website:
https://classic.clinicaltrials.gov/ct2/show/study/NCT04620538
Sponsor: Imperial College London
Funding: PhD Studentship
Background
Primary liver cancers (hepatocellular carcinoma and cholangiocarcinoma) do not usually cause symptoms in the early stages, therefore early diagnosis of liver cancer presents a significant challenge. Liver cancer is often, therefore, diagnosed at a late stage when curative treatment options are limited. As a result of late diagnosis, 5-year net survival in the UK is just 13%.
There is currently no screening programme available for liver cancer in the UK, however given that over 80% of liver cancers occur in the presence of cirrhosis, at-risk patients with cirrhosis or chronic hepatitis B or C are recommended to undergo a liver ultrasound and alpha fetoprotein (AFP) blood test every 6 months to improve identification of hepatocellular carcinoma.
Unfortunately, many patients are unaware they have cirrhosis so miss this opportunity, and for those that are aware attendance at follow-up investigations is frequently poor. Patients with primary sclerosing cholangitis (PSC) are also recommended to undergo surveillance for cholangiocarcinoma, but more accessible methods to monitor cirrhosis and PSC are required to help to improve early detection of these primary liver cancers.
To overcome the challenge of earlier detection of liver cancer, we propose a simple-to-use, non-invasive breath test to identify patients at risk of liver cancer, thus facilitating a streamlined referral for these patients to the appropriate specialised tests. The proposed test is based on the detection of volatile organic compounds (VOCs), which are carbon-containing compounds that are sufficiently volatile to be detectable in the gas phase at room temperature and can be found in exhaled breath.
VOCAL1
Participating Centres:

Aims:
1. Identify volatile biomarkers of hepatocellular carcinoma and cirrhosis.
2. Develop a diagnostic model for detection of hepatocellular carcinoma based on identified biomarkers.
Methods:
The VOCAL1 discovery study recruited 154 participants between March 2021 – April 2022. This included 39 participants with hepatocellular carcinoma, 68 participants with cirrhosis, and 47 controls (patients referred for a Fibroscan due to concerns regarding liver disease but ultimately found to have no evidence of cirrhosis based on Fibroscan score and clinical consensus).
Breath was collected using a standardised procedure and transported to our Imperial College London VOC Laboratory using thermal desorption (TD) tubes. Breath samples were analysed using gas chromatography-time of flight mass-chromatography (GC-MS-TOF).
VOCAL2
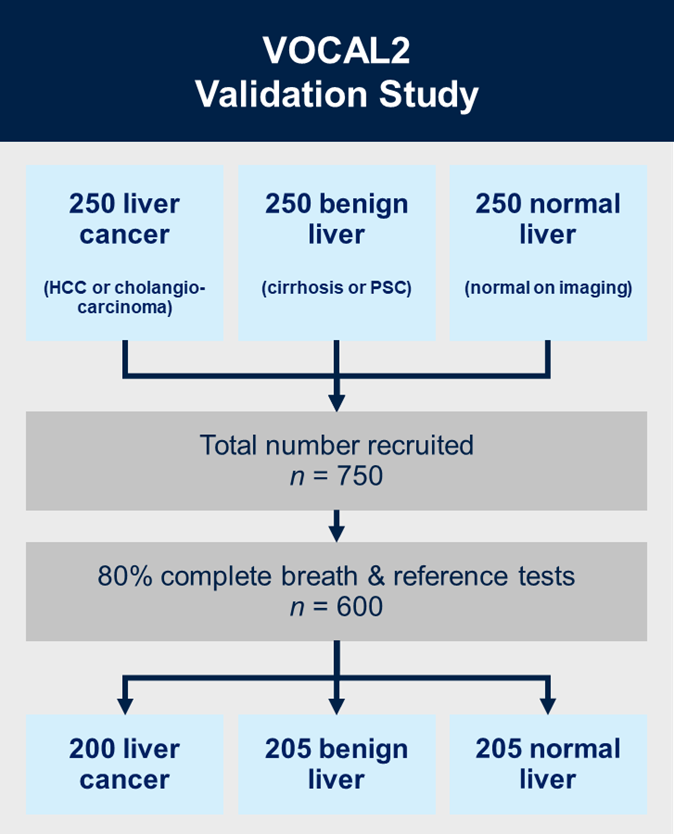
Aims:
1. Validate VOC biomarkers for liver cancer that were identified in VOCAL1 study.
2. Further discovery of VOC biomarkers for liver cancer in VOCAL2 study, including those for cholangiocarcinoma
3. Refine the diagnostic model for primary liver cancer with internal validation.
Methods:
VOCAL2 is a prospective multicentre, case-control, prediction study with internal validation. We will recruit 750 patients in total across three groups, each containing 250 patients: (i) liver cancer (hepatocellular carcinoma (HCC) or cholangiocarcinoma); (ii) liver cirrhosis or primary sclerosing cholangitis (PSC) without cancer; and (iii) radiologically normal liver in patients presenting with non-specific gastrointestinal symptoms.
Breath tests will be collected following a standardised protocol across all sites, and will be transported to the Imperial College London VOC Laboratory for analysis using TD-GC-TOF-MS using both polar and mid-polar columns, as well as 2D-TD-GC-TOF-MS for structural confirmation of compounds.
Participating centres:

Contact
For more information relating to the study, or if you are a healthcare professional interested in your centre participating in this study, please get in touch with Georgios Karagiannidis, via email: vocal-study@imperial.ac.uk.
